EPA Method 1682
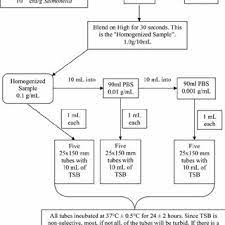
Three 400-g replicates of each compost sample were placed into Ziploc (SC Johnson & Sons, Racine, WI) bags and inoculated with 7 mL of the multistrain inoculum of E. coli , Salmonella spp., and E. coli O157:H7, yielding an approximate population of 10 1–2 CFU/g per organism. Im- mediately after adding inocula to the compost sample, the bag was sealed and the sample was manually homogenized for 5 min. Each compost replicate was then processed ac- cording to the protocols as described in Figures 1–4. Figure 1 (EPA Method 1680) and Fig. 3 (TMECC 07.01) illustrate the recovery methods used for fecal coliforms. After EPA Method 1680 was completed, 10 l L of culture from EC tubes that were positive for fecal coliforms were isolated on MACR to recover the rifampicin-resistant nonpathogenic E. coli inoculated into the compost (to directly compare results to the TMECC 07.01 method).
TMECC 07.02 [B and C] show the methods used for the recovery of Salmonella spp. Uninoculated compost from each of the 29 samples were analyzed for the following characteristics using standard laboratory methods (Table 3): carbon:nitrogen ratio (C:N); total organic carbon (TOC); % moisture; % volatile solids; pH; electrical conductivity (EC); and maturity (tested by CO 2 and NH tests and maturity index [Solvita, Mt. Vernon, ME]). Ten grams of inoculated compost were combined with 90 mL tryptic soy broth (TSB) in a filtered WhirlPak bag (Nasco, Ft. Atkinson, WI) and homogenized for at least 30 s.
Homogenates were spiral-plated (200 l L, in duplicate) onto CHROMN, which were incubated at 37 ° C for 24 – 2 h before populations were determined and expressed in CFU per gram (dry weight) for analyses. All 29 (27 for E. coli O157:H7) inoculated compost samples were simultaneously assayed by all 5 described recovery methods in a completely randomized factorial design. The recovery percentages of E. coli and Salmonella determined by the EPA
There are no products listed under this category.