Organochlorine Pesticide Analysis
Organochlorine Pesticide Analysis using HRGC/MS/MS
Organochlorine pesticides (OCPs) have a long history of use in the United States and around the world. Although the production and use of many OCPs has been banned since the 1970s, the compounds are extremely persistent in the environment and are known for accumulating in sediments, plants and animals. OCPs have a wide range of both acute and chronic health effects, including cancer, neurological damage, and birth defects. Many OCPs are also suspected as endocrine disruptors.

Due to the need to monitor levels of OCPs, significant research and development has taken place over the last 40 years. This has culminated in two primary methods used by the Environmental Protection Agency (EPA) to regulate the transport and fate of OCPs; EPA Method 1699 and EPA Method 8081.
EPA Method 1699, isotope dilution and high-resolution mass spectrometry, is considered the ultimate in pesticide analysis relative to sensitivity and selectivity, but can be cost prohibitive.
EPA Method 8081 is a gas chromatography method that employs electron capture as a means of detection. To achieve some level of selectivity, the method is run in dual column mode with two dissimilar analytical columns. It has been well documented that the use of ECD can lead to false positives or high biases in the results generated by this method.
This lack of selectivity is especially apparent when polychlorinated biphenyls (PCB) are present in the sample.
Table 1
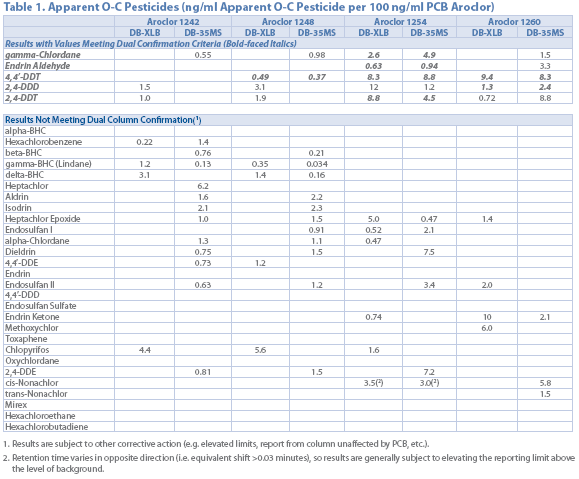
Table 1 lists the apparent concentration of pesticides when a 100 ng/mL PCB standard in analyzed using EPA Method 8081. While quantifying the interferences can help in data interpretation, a more selective and robust alternative is needed.
Goals for the for the determination of OCPs by GC/MS/MS
• Provide a lower cost alternative to GC/HRMS
• Achieve lower reporting limits than GC-ECD
• Maximize selectivity using two transitions per analyte
• Use isotope dilution quantitation
GC/MS/MS Instrumentation
The key to achieving the selectivity needed to eliminate PCB and other co-extractable interferences is to operate the tandem mass spectrometer in the Multiple Reaction Monitoring (MRM) mode.
MRM Mode
• Precursor ion selected with the first quad (MS1)
• Ion enters the collision cell where fragmentation occurs
• Compound-specific fragment ions are selected with the second quad (MS2) and then detected
The MRM operating mode is depicted in Figure 1.
This ionization of a precursor to a product mass is called a transition.Two unique transitions are acquired for each analyte of interest. The ratio of the two transitions is then used to set criteria to increase confidence in analyte identification of unknown samples. The MRM mode is also inherently more sensitive because the signal to noise ratio is increased.
Materials & Methods
Sample Preparation
• All samples are spiked with labeled isotopes prior to extraction
• Solid samples are prepared using EPA Method 3541- Automated Soxhlet Extraction
• 10 grams for soils, sediments and tissues
• 1 mL final extract volume
• Liquid samples prepared using EPA Method 3535 – Solid Phase Extraction
• One liter sample size
• 1 mL final extract volume
• Prior to sample analysis tissue extracts are subject to lipid removal using Supleco lipid removal agent. Soil/sediments samples are subject to carbon cleanup. Alternate cleanups include GPC and Florisil®
• Method Detection Limit studies were prepared for water, soil and tissue matrices
Standards
• Individual pesticide standards, Accustandard, Inc.
• Labeled isotope standards, Cambridge Isotope Laboratories, Inc. and CDN Isotopes.
• Internal standard of PCB52 (13C1299%), Cambridge Isotope Laboratories, Inc.
• Each standard and sample is spiked with 100 ng/mL of internal standard prior to analysis.
• A calibration curve was then prepared at a nominal concentration of 0.5 ng/mL to 100 ng/mL.
Injector Parameters
• Agilent PTV
• Mode Solvent Vent, Ramped pressure
• Injector Liner: Baffled, Restek Siltek Deactivated; #21704-214-10
• Injection Volume: 5 μl
• Injection speed: Slow
• Post injection dwell: 1.0 min
• Carrier gas: Helium @ 0.7 mL/min
• Injector temperature: 40-320°C, temperature programmed
GC/MS Run Conditions
• GC: Agilent 6890N
• Mass Spec: Waters Micromass Quattro Tandem MS
• Column: Phenomenex ZB-1, 30m x 0.25mm x 0.25μm
• Oven: 65°C initial temp for 2.0min
• 20°C/min to 240°C for 0.0min
• 6.1°C/min to 280°C for 0.0min
• 15°C/min to 310°C for 0.7min
• Run Time – 20 minutes
• Source temp: 225°C
• Detector interface temp: 250°C
• Collision gas: Argon @ 2-3 x 10-3 torr
Results & Discussion
The chromatography conditions yielded good resolution of OCPs by GC/MS/MS as the reconstructed ion chromatograph of the MS/MS acquisition shows in Figure 2. While peak resolution isn’t as critical as in GC/ECD analysis, it is still required to separate isobaric interferences and to maximize dwell time for optimal sensitivity.
The response of the low calibration point of 0.5 ng/mL produced excellent signal to noise ratios as shown in Figure 3. The calibration of each pesticide showed good linearity with most under 10% RSD, and all within EPA criteria. Table 2 summarizes calibration results of each analyte.
Acceptable method detection limits studies were analyzed for water, soil and tissue matrices and are summarized in Table 3. All method detection and reporting limits exceed the sensitivity of GC-ECD and are about an order of magnitude above high-resolution MS limits.
To assess selectivity a 5000 ng/mL standard of Aroclor 1254 was acquired under the MS/MS method. Note this level is 50X higher than the standard that gives false positives by GC-ECD. The results are summarized in Table 4.
To further assess selectivity, SRM 1945 (Whale Blubber) was analyzed and compared against GCECD. The results are summarized in Figure 4. Overall the GC/MS/MS analysis of SRM 1945 produced solid recoveries against the true values. It again shows superior selectivity when comparing results for cis-Nonachlor, 2,4’-DDD, 2,4’-DDT, 4,4’-DDT and Dieldrin, all of which had a high bias using GC-ECD for the tissue matrix due to the presence PCB congeners.
Conclusions
• GC/MS/MS provides superior performance of GC-ECD and a solid alternative to GC/HRMS
• Up 20X decrease in reporting limits over GC-ECD
• Significant increase in selectivity operating in MRM mode using two transitions per analyte
• Cost of analysis is less than half of GC/HRMS
References
1. D. Muir, E. Sverko, Analytical Methods for PCBs and Organochlorine Pesticides in Environmental Monitoring and Surveillance: a Critical Appraisal, Anal Bioanal Chem, (2006) 386:769-789.
2. L. Jones, M. Tritt, A Unique Approach for Evaluating Aroclor Interferences of Chlorinated Pesticides in Tissue for Portland Harbor Superfund Site, Poster Presentation SETAC, 2005.
3. Waters Quattro Micro™ GC Applications Training Documentation, Chapter 1, March, 2008.
There are no products listed under this category.