InvBio Saliva 20 Tests ( without going into nose)
Invbio
Order Online On maxanim.com
- SKU:
- INVBIO-IOV87953
- Condition:
- New
- Availability:
- We ship within 8 hours of receiving your order. Next day delivery of all Invbio Tests except for weekends.
Order Online On maxanim.com
The InvBio C2 Antigen Rapid Test Device (Saliva)
- REF: IOV87953
- For professional in vitro diagnostic use only.

No shwab is used to obtain this result! The easiest test in 2022 for Omicron and Delta security!
Expected Usage
The INVBIO Cov Antigen Rapid Test Device (Saliva) is a polymer immunochromatographic technology and double antibody sandwich principle that is intended for the qualitative detection of the N protein antigen from SARS-CoV-2 in human saliva specimens directly. This N protein is present in the Omicron, Delta and all older former variants.

The Saliva cone is a very easy and non invasive method to test small children. Also it is easier for the tester to not come in contact with the saliva fluid. This is the easiest and fastest way of testing large amounts of people.
Testing is limited to laboratories, airports, medical institutions, events, schools and businesses.
Results are for the identification of N protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of transmission. Positive results indicate the presence of viral antigens.
- Positive results mean 7 days of quarantine for the tested person.
- Negative results should be treated as presumptive and confirmed with a molecular PCR assay if necessary.
- Negative results do not rule out C19 and should not be used as the sole basis for treatment or management decisions.
- Negative results should be considered in the context of a person’s recent exposures, history and the presence of other signs.
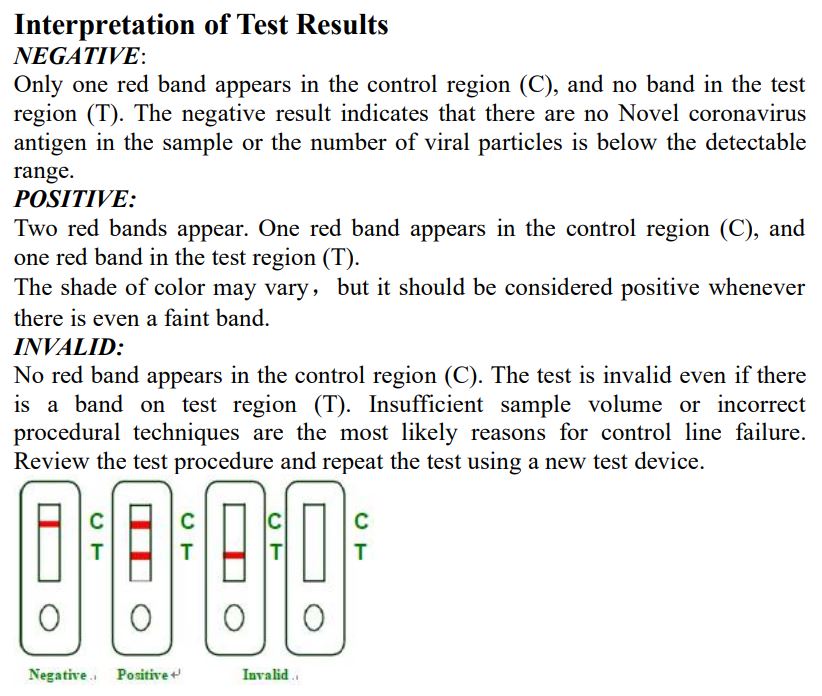
The INVBIO COVID-19 Antigen Rapid Test Kit is intended for use by a tester to also test asymptomatic infected people that also can be an infectious source. Based on the current investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days.
The main manifestations of omicron include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
The polymer immunochromatographic technology and double antibody sandwich principle were used to detect the novel c2 antigen in human saliva specimens with the principle of capture method. During the test, a specimen solution is added to the sample well of the kit. The specimen is first mixed with the colored polymer-labeled c2 monoclonal antibody 1 on the release pad, and then chromatographed on a nitrocellulose membrane. If the specimen contains c2 antigens, these antigens will first bind to colored polymer-labeled c2 monoclonal antibody 1, so that when the mixture is chromatographed on a nitrocellulose membrane, it will be immobilized with the c2 monoclonal antibody 2. The detection line (T line) was captured to form a colored polymer-labeled c2 monoclonal antibody1-antigen- c2 monoclonal antibody 2 immune complex. Therefore, a red line appeared on the T line, which was a positive result.
If no viral antigen is present in the specimens of the subject, a red line will not be formed on the test line (T line), which is a negative result. The quality control line (C line) on the test cassette is coated with goat anti-mouse antibody. Under normal circumstances,a red line should appear on the quality control line(C line) during the test to prove that the test cassette is working properly.
Main Ingredients
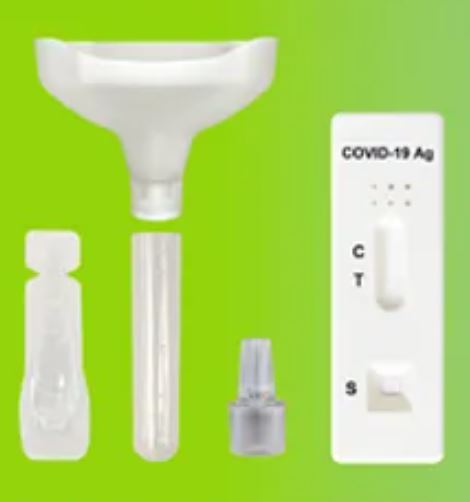
1.Material Provided:
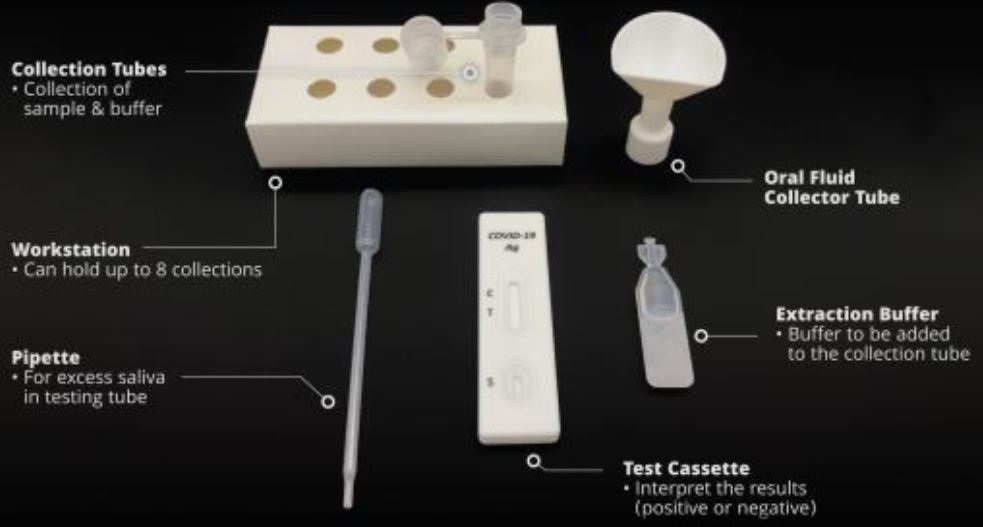
- 20 Test Cassettes
- 20 Saliva Collectors ( Cones for spitting )
- 20 Collection Tubes
- 20 Specimen Extraction Buffers
- 20 Droppers
- 1 Work Station
- 1 Instructions Manual in Geman, English or Dutch

2.Material required but not provided:
Timer. ( the 15 minutes time factor is very wide so without timer will work too )
InvBio test Storage Conditions and Stability
- Store the C2 Antigen Rapid Test Device (Saliva) at 2-30°C.
- Do not freeze.
- All reagents are stable until the expiration dates marked on their outer packaging and buffer vial.
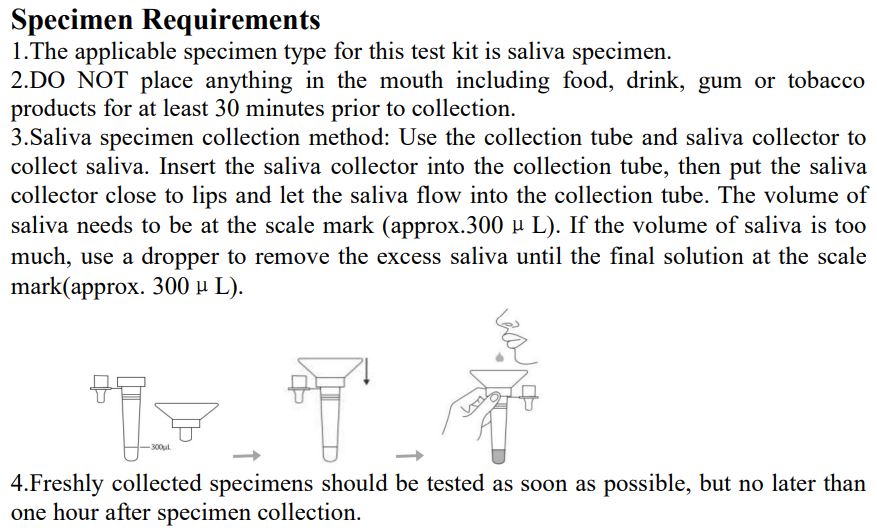
Specimen Requirements
1.The applicable specimen type for this test kit is saliva specimen.
2.DO NOT place anything in the mouth including food, drink, gum or tobacco products for at least 30 minutes prior to collection.

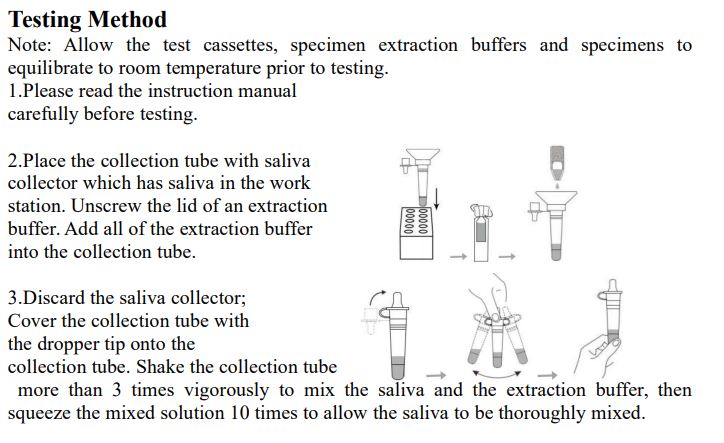
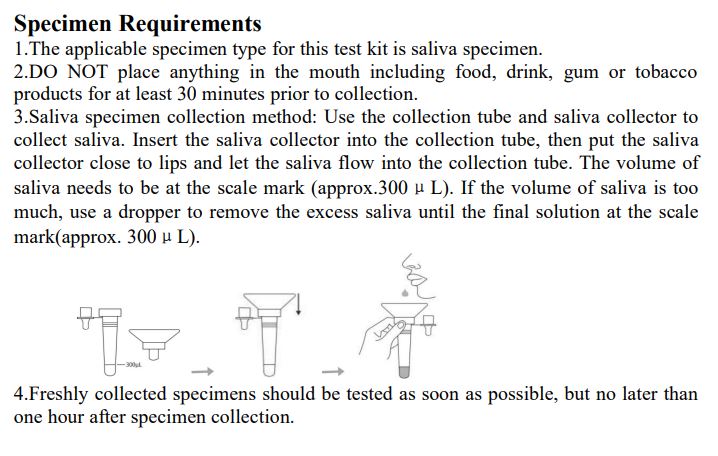
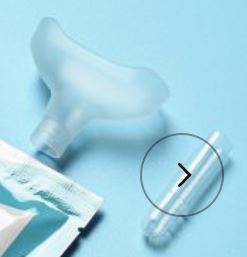
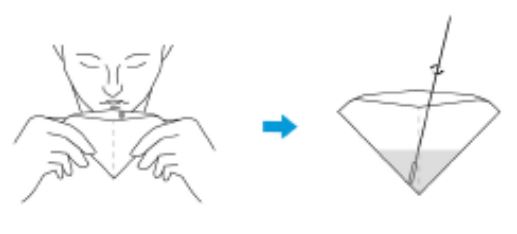
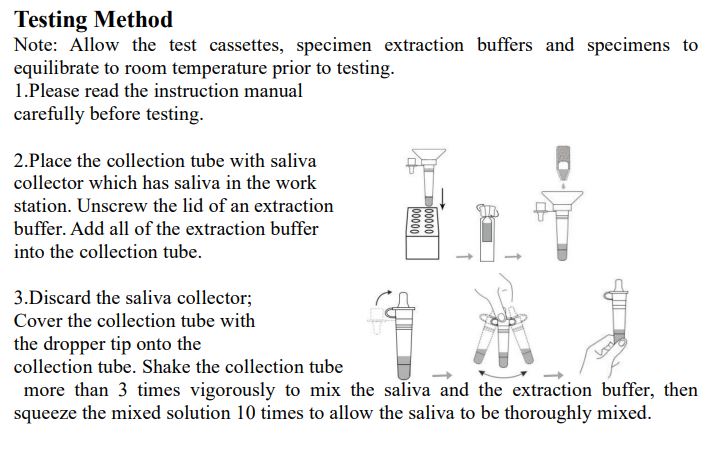
3.Saliva specimen collection method: Use the collection tube and saliva collector to collect saliva. Insert the saliva collector into the collection tube, then put the saliva collector close to lips and let the saliva flow into the collection tube. The volume of saliva needs to be at the scale mark (approx.300μL). If the volume of saliva is too much, use a dropper to remove the excess saliva until the final solution at the scale mark(approx. 300μL).
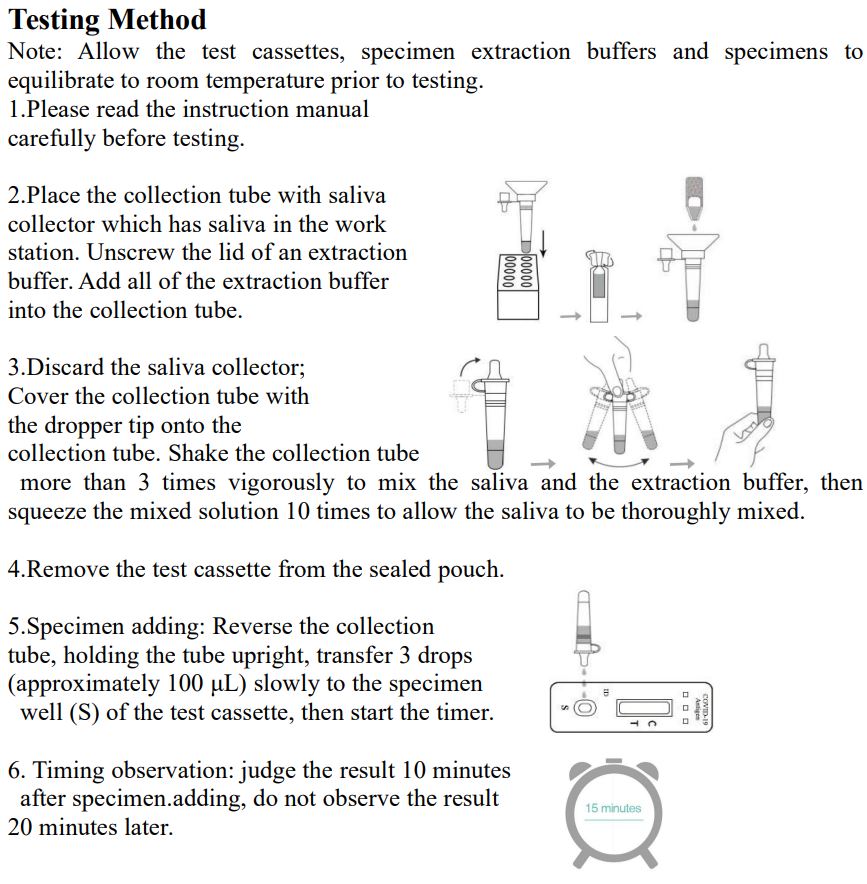
4.Freshly collected specimens should be tested as soon as possible, but no later than one hour after specimen collection. Testing Method Note: InvBio test cassettes only.
Lieven Gevaert Bio-Ir.
Mobile 0032 476 375 875
The author has 5 children and they test them selves every day after school because they like to go to school safe. The school has known lockdowns due to no or late testing. The result of a PCR test could take up to 24 - 48 hours while a rapid self test will take only 15 minutes.
A self test can be done every day whole a PCR test is to costly to perform it every day.
It is true that a PCR test will detect earlier positive samples but if taken only from visible ill persons and with the long waiting time it is proven less effective than the easy, non invasive, low cost InvBio saliva tests that are daily used on all of the children.

Gentaur Bv
Voortstraat 49
1910 Kampenhout BELGIUM
Tel 0032 16 58 90 45
Fax 0032 16 50 90 45
lieven@gentaur.com
2 Reviews Hide Reviews Show Reviews
-
good support
I highly recommend it. It is easy to use and you get your results really fast.
-
Quick shipping
I am very pleased with the service I received!