UnScience Antigen Test
Antigen Rapid Test Kit
This Antigen Rapid Test Kit (Colloidal Gold) uses the sandwich immunocapture method and colloidal gold immunochromatography to qualitatively determine the presence of antigens in human oropharyngeal swabs, nasal swabs and nasopharyngeal swabs. It is helpful as an aid in the screening of early mild, asymptomatic, or acute patients for identification of infection.
Certified with ISO 13485 , CE and other documents
Suitable for: point-of-care use, community, remote regions
Sensitivity: 96.330% (95% CI: 90.870%, 98.991%)
Specificity: 99.569% (95% CI: 97.622%, 99.989%)
Features of Rapid Antigen Test
- Easier: No special equipment needed; Easy to use; Intuitive visual interpretation.
- Rapid: Results in 10 minutes.
- Accurate: Results were validated by PCR and Clinical diagnosis.
- Diversity: Works with oropharyngeal swab, nasal swab and nasopharyngeal swab.
How Does the Antigen Rapid Test Kit (GICA) Work?
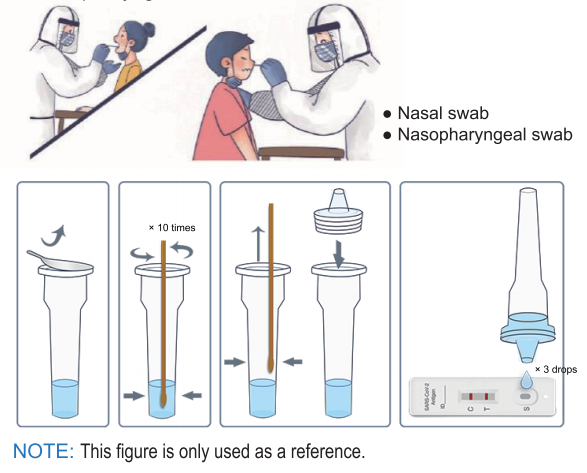
Before use, please read the instructions carefully and operate in strict accordance with the instructions:
- 1.Bring the pouch to room temperature before use.
- 2.Take out the cassette, put it on a horizontal table.
- 3.Add 3 drops of the processed sample vertically into the sample well and start the timer.
- 4.Observe the result after 10 minutes, the result is valid within 30 minutes, read results after 30 minutes is invalid.
Testing Results
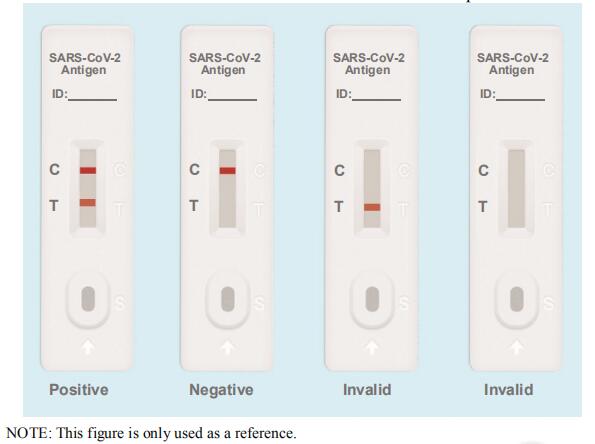
- Positive: Both the detection line (T line) and the quality control line (C line) appear colors.
- Negative: The test line (T line) does not appear color, only the quality control line (C line) appears color
- Invalid: The quality control line (C line) does not appear color, which means that the test is invalid and the test should be repeated.
Materials supplied:
- 1.Test reagent: 1 test / pouch, each test consists of a test cassette and a desiccant. The cassette is composed of a test strip and a test strip shell. The test strip consists of a sample pad and a colloidal gold bonding pad (sprayed with colloid Gold-labeled monoclonal antibody I), nitrocellulose membrane (NC membrane) (the detection area is coated with monoclonal antibody II (T line) and goat anti- Mouse IgG (C line)), liner and absorbent pad.
- 2.Desiccant: 1 piece / pouch, silica gel.
- 3.Swab: 25 pieces / pack.
- 4.Sample treatment solution: 25 vials / pack.
- 5.Tube cap: 25 pieces / pack.
Storage and Stability:
The test reagent is stored at 2 ~ 30 ℃, and the validity period is tentatively set for 18 months.
See the label for the production date and expiration date.
Limitations of Antigen Rapid Test Kit (Colloidal Gold)
- 1.This kit is a qualitative test for in vitro auxiliary diagnosis.
- 2.Due to methodological limitations, the sensitivity of this kit is lower than that of PCR. Therefore, more attention should be paid to the negative results of this experiment, and a comprehensive judgment should be combined with other test results. It is recommended that the suspected results be supplemented with nucleic acid testing or virus isolation and culture in vitro for confirmation.
- 3.Unreasonable sampling, transportation and handling, or low virus content in the sample will lead to false negative results.
- 4.The test results of this reagent are for clinical reference only and cannot be used as the only basis for clinical diagnosis. The tester should conduct a comprehensive evaluation based on the patient's clinical manifestations and other laboratory test results.
About UNscience
UNscience (https://www.uni-science.com) , a wholly owned subsidiary of Elabscience, specializes in the research and development, production and sale of in-vitro diagnostic reagents. Certified with ISO 13485 and other documents, UNscience sell its products all over the world.
The company has 100,000 grade GMP purification workshop and quality management system, three major technical platforms (Colloidal Gold Immunochromatographic Platform, Fluorescence Immunochromatographic Platform, and Pathological Diagnosis Antibody Platform). UNscience has the independent research and development and production capacity of core raw materials, and has successfully developed 27 POCT immunochromatographic quantitative detection products (colloidal gold and fluorescence), mainly covering cardiovascular and cerebrovascular diseases, kidney diseases, diabetes, infectious diseases, reproductive health, health examination and other.