Roche Antigen Test Nasal (25 Test Box)
Roche
Order Online On maxanim.com
- SKU:
- 09365397023
- Availability:
- IN STOCK
Order Online On maxanim.com
The Roche Rapid Antigen 25 Test box for Nasal
- Reliable lateral flow casette for nucleocapsid antigen present in human nasal samples
- The nucleocapsid of the 2022 Omicron variant is detected by this test as well as the order delta and older variants.
The Bulk prices for this Roche tests are 4800 tests for only 18240 €!
Caslab Gentaur offers the lowest price for smal and large orders, delivered next day or in maximum 3 working days.
( We suggest not to order more than 4 palettes in one order 72960 € )
Catalog numbers
- 09365397023 SARS-CoV-2 Rapid AG Test Nasal 25T: €95 (3.8€/test)
- 09327592023 SARS-CoV-2 Rapid AG Test 25T. Variant 1: €95 (Nasopharyngeal) : (3.8€/test)
The Roche Nasal Rapid Antigen Test is a rapid shallow antigen test in the front of the nose.
Roche supplies the best chromatographic lateral flow Immunoassay for the Qualitative Detection of Nucleocapsid Antigen Present in Human Nasal Samples
- Approved as SELF-TEST in all Eurpean counties
- Very fast result after 12 minutes (display window 15-30 minutes).
- Easy operation (take through nasal swab).
- No further instruments nor lab needed.
- The test kit is ready to use and contains everything you need to perform a test.
- They can only be ordered per kit of 25 pieces.
Reference Manufacturer: Roche-09365397023

Categories: ENT examination
- SHALLOW nasal test Roche Antigen Test 25 pieces in a box
- Rapid Nose Antigen Test
- Approved SELF-TEST
- Omicron and Delta Nucleocapsid Antigen Present in Human Nasal Samples
- Can be used on veterinary samples of cat and ferret
Roche guerantees Simple Sampling and Fast Results
This Roche Test Nasal provides rapid results from collection from the front of the nose from swab samples that can be taken by patients themselves, providing better protection for healthcare professionals.
The Roche Nasal test protocol:
- The nasal collection is less stressful for children, the older or people with disabilities
- The test pack is ready to use, has pre-filled extraction buffer tubes and contains everything you need to perform a test
- No further materials needed
- Simple operation and result after 12 minutes (display window 15-30 minutes) – no follow-up appointment necessary
- Storage at room temperature (+1 – +32°C)
- Enables decentralized testing and access to testing in areas where lab testing is unavailable
- Less chance of sneezing and coughing during the administration. The controlled self-sampling prevents physical contact
Roche points out that the Health and Youth Care Inspectorate (IGJ) has indicated (22/10/20) that involvement of a doctor with a BIG registration as medically responsible person for the proper administration of the tests, the interpretation of the test result and the passing on positively tested persons to the GGD is very important.

The Rapid Antigen Test Nasal from Roche should always be performed by a professional
Roche Diagnostics believe that broad access to such high-quality and reliable, instrument-less Point of Care tests can contribute significantly to better management of the current pandemic crisis.
Features and Benefits of Roche's Test Nasal
- Compared to the existing Rapid Antigen Test from Roche, the sample for the SARS-CoV-2 Rapid Antigen Test Nasal is collected from the front of the nose instead of deep in the nose, resulting in a simplified and faster testing procedure.This testing method can help reduce overall patient discomfort, especially in sensitive individuals such as children, the elderly, and/or people with disabilities.
- Enables decentralized testing or access to testing in areas where lab testing is unavailable
- Test kit information The kit is ready to use and contains everything you need to perform a test.
The following parts are required for a test and are included in the kit:
- Roche Test device (individually wrapped in foil with desiccant)
- Extraction Buffer Tubes and Buffer Tube Rack
- drop caps
- Sterile Nasal Swabs
- Roche's Positive and Negative Control
- The quick and easy test
- Test Protocol of the Rapid Antigen Test Nasal

1a. Collection of the nasal sample from the left and right nostril
Testing process
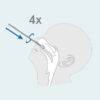
- Insert the sterile swab into the nostril with the most discharge.
- While twisting the swab, insert the swab 2cm parallel to the palate (not up) toward the throat into the nostril until you feel resistance at the turbinates.
- Do not apply pressure. Twist the swab against the nasal wall 4 times for about 15 seconds and remove it from the nostril.
1b.
- Repeat step 1a. with the same swab in the other nostril.
- Note: The sample should be taken from both nostrils with the same swab.
- Prepare a sample
2a.
- Place the swab in an extraction buffer tube.
Rock the swab back and forth more than 10 times while squeezing the buffer tube.
2b.
Remove the swab while squeezing the sides of the tube to extract the liquid from the swab.
WARNING! Failure to squeeze the tube may cause incorrect results due to excess buffer in the swab.
2c.
Press the spout cap firmly onto the tube. Continue with 3a. Run a test.
3a.
Place the Tester on a flat surface and place 4 drops of the extracted sample at a 90 angle into the sample well of the Tester.
3b.
Read the test result after 15 to 30 minutes.
WARNING! Risk of incorrect results. Do not read the test result after 30 minutes.
Interpreting results
Testing process
4.
A colored line appears in the top portion of the results window to indicate that the test is working properly. This is the control line (C). Even if the control line is faint, the test should be considered to have been performed correctly. If no control line is visible, the test is invalid.
If the result is positive, a colored line also appears in the lower part of the result window. This is the test line (T). Even if the test line is very faint or uneven, the test result should be interpreted as a positive result.
The Roche Rapid Antigen Test Nasal provides rapid results for fast decision making at the Point of Care. Nasal sampling is less invasive, which can reduce patient discomfort, and minimize the duration of the procedure as well as the risk of sneezing or coughing. Nasal swab samples may be self-collected by patients under supervision, offering further protection for healthcare professionals.
Antigen-test-nasal-kit advantages
- Simple sampling through a nasal swab (lower nasal area)
- Getting a quick result within 15-30 minutes
- No need for a follow-up appointment to discuss the result
- Self Sampling: Sample taken by the patient (under professional supervision)
- Simple test execution, no pipetting steps, no complex processes
- High quality
- Samples taken by qualified personnel: relative sensitivity: 90.6%, relative specificity: 98.6%
Samples taken by patients: relative sensitivity: 84.4%, relative specificity: 99.2%
Known mutations (UK, South Africa, Brazil) do not affect the test performance
For symptomatic as well as asymptomatic subjects
No influence from mRNA vaccines (Pfizer, Moderna)
Accessible - high-performance, instrument-free and reliable Point of Care test
Allowing decentralized testing or access to testing in areas where laboratory testing is not available
Providing patients with the option to self-collect their nasal sample
Testing the quick and easy way
Testing process for the SARS-CoV-2 Rapid Antigen Test Nasal
SARS-CoV-2 Rapid Antigen Test Nasal handling video
videoImage
Test kit information
The kit is ready for use and contains all equipment needed to perform a test.
The following components are needed for a test and included in the kit:
Test device (individually in a foil pouch with desiccant)
- Extraction buffer tube and buffer tube rack
- Nozzle cap
- Sterile swab
- Instructions for use and Quick Reference Guide
- Positive and negative controls
- SARS-CoV-2-Antigen-test-nasal-kit
- Collecting the nasal sample from left and right nostril
References
- Su S, Wong G, Shi W, et al.-Trends Microbiol. 2016;24(6):490–502.-2016-Elecsys Anti-SARS-CoV-2 -L (v1.0)
- Zhu, N., Zhang, D., Wang, W. et al.-N Engl J Med 382(8) 727-733-2020-Elecsys Anti-SARS-CoV-2 -Lit (v1.0)
- Chan, J.F., Yuan, S., Kok, K.H., To, K.K., Chu, H., Yang, J. et al.-Lancet. 395, 514–523.-2020- El (v1.0)
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. Published April 2, 2020. Accessed February 5, 2021.
- WHO. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Published March 29, 2020. Accessed February 5, 2021
- Kampf, G., Todt, D., Pfaender, S., Steinmann, E.-J Hosp Infect. 104(3), 246–251.-2020- Elecsys Ant (v1.0)
- Letko, M., Marzi, A., Munster, V. (2020).-Nat Microbiol. 1–8. doi:10.1038/s41564-020-0688-y.-2020- (v1.0)
- Hoffmann, M., Kleine-Weber, H., Schroeder, S. et al.-[published online ahead of print, 2020 Mar 4]. (v1.0)
- WHO. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200403- sitrep-74-covid-19-mp.pdf. Published April 3, 2020. Accessed April 15, 2020.
- Lauer SA et al.-Ann Intern Med 2020;172(9):577-82-The Incubation Period of COVID-19 (v1.0)
- Rothe, C et al-N Engl J Med 2020;382(10):970-971-2020-Lab Infectious Diseases Respiratory tract infec (v1.0)
- Kupferschmidt K-Science. https://www.sciencemag.org/news/2020/02/paper-non symptomatic-patienttrans (v1.0)
- Bai Y et al-JAMA 2020;323(14):1406-1407-2020-Lab Infectious Diseases Respiratory tract infections Co (v1.0)
- Mizumoto K et al-Euro Surveill 2020;25(10):2000180.-2020-Lab Infectious Diseases Respiratory tract (v1.0)
- SARS-CoV-2 Rapid Antigen Test Nasal Package Insert 2021-01 V 1.0
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Published March 20, 2020. Accessed April 15, 2020.
- Wang, D. et al. (2020). JAMA. 323(11), 1061-1069.
- Huang, C. et al. (2020). Lancet. 395(10223), 15-2.
- Arentz, M. et al. (2020). JAMA. 323(16), 1612-1614.
- Wu, Z. et al. JAMA. 323(13), 1239-1242.
- WHO. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19- laboratory-2020.5-eng.pdf. Published March 19, 2020. Accessed April 15, 2020.
- U.S. CDC. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published March 14, 2020. Accessed April 15, 2020.
- EUCDC. https://www.ecdc.europa.eu/sites/default/files/documents/Overview-rapid-test-situation-for-COVID-19-diagnosis-EU-EEA.pdf. Published April 1, 2020. Accessed April 15, 2020.
- WHO. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/. Published April 11, 2020. Accessed April 15, 2020.
- Liu, W. et al. (2020). J Clin Microbiol. 58(6), e00461-2.
- To, K. et al. (2020). Lancet Infect Dis. 20(5), 565-74.
- Long, Q. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.18.20038018.
- Lou, B. et al. (2020). Eur Resp J. https://doi.org/10.1183/13993003.00763-2020.
- Zhao, J. et al. (2020). Clin Infect Dis. pii: ciaa344. https://doi.org/10.1093/cid/ciaa344.
- Zhang, B. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.12.20035048.
- Wölfel, R. et al. (2020). Nature. 581, 465-469.
- Xiao, D.A.T. et al. (2020). J Infect. 81(1), 147-178.
- Tan, W. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.24.20042382.
- Okba, N. et al. (2020). medRxiv. https://doi.org/10.1101/2020.03.18.20038059.
- Alberts, B. et al. (2002). Molecular Biology of the Cell. 4th edition. New York: Garland Science. B Cells and Antibodies. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26884/
- Klasse, P.J. (2016). Expert Rev Vaccines 15(3), 295-311.
- Payne, S. (2017). Viruses: Chapter 6 - Immunity and Resistance to Viruses, Editor(s): Susan Payne, Academic Press, Pages 61-71, ISBN 9780128031094.
- Iwasaki, A. and Yang, Y. (2020). Nat Rev Immunol. https://doi.org/10.1038/ s41577-020-0321-6.
- Amanat, F. et al. (2020). Nat Med. https://doi.org/10.1038/s41591-020-0913-5.
- Zhou, P. et al. (2020). Nature. 579(7798), 270-273.
- Haveri, A. et al. (2020). Euro Surveill. 25(11), 2000266.
- Poh, C. et al. (2020). bioRxiv. preprint doi: https://doi.org/10.1101/2020.03.30.015461.
1 Review Hide Reviews Show Reviews
-
superb packing
It was very easy to use . I appreciated how quickly I got my results. I would recommend it.







