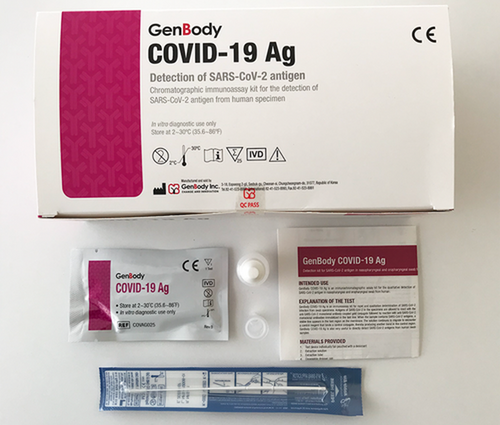
GenBody C-19 Antigeen Test Kits
- SKU:
- GB-C-19-Ag
- Size:
- 20 Tests
The GenBody C-19 Ag is a rapid immunochromatographic diagnostic test (RDT) intended for the qualitative evaluation Direct nasopharyngeal (NP) or anterior nasal (AN) CoV-2 nucleocapsid protein antigen detection swab samples from people suspected of having C-19 by their health care provider within the first six days from the onset of symptoms, or individuals without symptoms or other epidemiological reasons to suspect C-19 when tested twice over two to three days with at least 24 hours and no more than 48 hours between testing Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, who meet the requirements to take tests of moderate, high, or waived complexity. Is the product is authorized for use at the point of care (POC), that is, in patient care settings operating under a CLIA.
Certificate of Waiver, Certificate of Compliance or Certificate of Accreditation. The results are for the identification of the CoV-2 nucleocapsid antigen. The antigen is usually detectable on nasopharyngeal (NP) or anterior nasal (AN) swab samples during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnoses the information is necessary to determine the infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The detected agent may not be the definitive cause of the disease. laboratories inside.
The United States and its territories must report all results to the appropriate public health authorities. Negative results should be treated as presumptive and can be confirmed with a molecular assay, if necessary. for patient management. Negative results do not rule out CoV-2 infection and should not be used as sole basis for patient treatment or management decisions, including infection control decisions. negative results should be considered in the context of the patient's recent exposures, history, and presence of clinical signs and symptoms consistent with C-19.
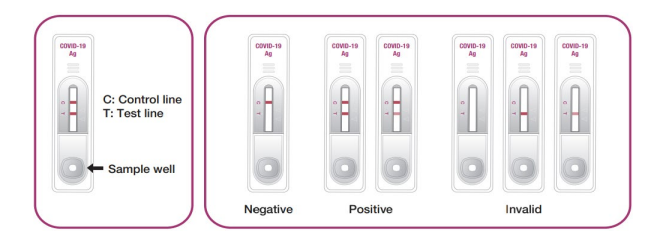
TEST EXPLANATION
GenBody C-19 Ag is an immunoassay kit for the rapid and qualitative determination of CoV-2 infection from swab samples. CoV-2 antigens in the samples are allowed to react with the anti-CoV-2 monoclonal antibody-coupled gold conjugate followed by reaction with immobilized anti-CoV-2 monoclonal antibodies on the staining line. proof. When the sample contains CoV-2 antigens, a visible line appears on the test region of the membrane. The solution continues to migrate to meet a control reagent that binds to a control conjugate, thus producing another band in the control region. GenBody C-19 Ag is also very useful for directly detecting CoV-2 antigens from human swab samples.
SUPPLIED MATERIALS
1. Individually bagged test device with a desiccant
2. Extraction solution
3. Extraction tube
4. Disposable dropper cap
5. Sterilized nasopharyngeal swabs for sample collection
6. Sterilized oropharyngeal swabs for sample collection (optional)
7. Instructions for use
MATERIALS REQUIRED BUT NOT PROVIDED
1. Medical mask and medical latex gloves.
2. Sample collection container
3. Micropipettes and disposable pipette tips
4. Clock or stopwatch
PRECAUTIONS
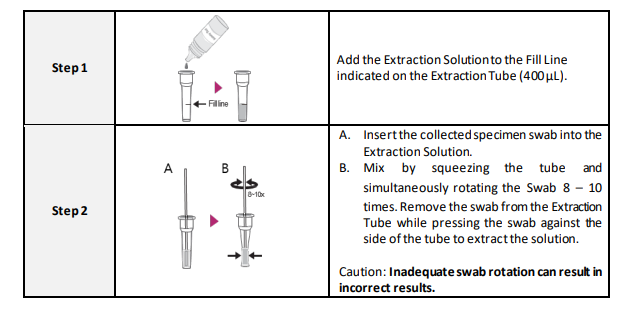
1. The presence of moisture can decrease the stability of the reagents. Therefore, perform the test immediately after removing the device from the foil bag.
2. For in vitro diagnostic use only. DO NOT reuse the test device.
3. The collected specimen should be prepared as a sample according to the "Specimen Collection and Storage" mentioned below and tested as soon as possible.
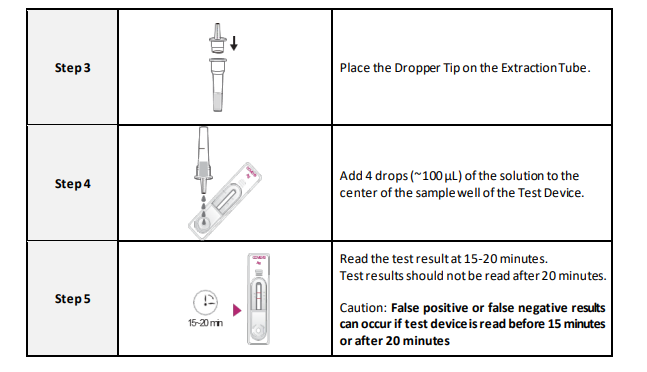
4. Add the fixed volume (4 drops) to the center of the sample well area.
5. Bring test kit and extraction solution to room temperature (15-30°C) prior to testing (15-30 min).
6. Keep interpretation time because it causes false negatives and false positives.
7. When using viral/universal transport media samples, inaccurate results may occur due to decreased sensitivity of the test.
8. When using transport media for sample collection, DO NOT use Nucleic Acid Storage and Transport (NAPT) medium.
SAMPLE COLLECTION AND STORAGE
1. Specimens to be tested must be obtained and handled using standard collection methods.
2. Nasopharyngeal swab sample: To collect a nasopharyngeal sample, carefully insert the sterile swab into the nostril with the greatest amount of discharge under visual inspection. Using gentle rotation, push the swab in until it meets resistance at the level of the turbinates (less than an inch into the nostril). Rotate the swab several times against the nasal wall.
3. [Optional] Oropharyngeal swab sample: Insert the oral cavity swab into the pharynx slowly, and collect the epidermis of the mucous membrane by swabbing the posterior pharyngeal wall or facial tonsil several times. The antigen in sufficient quantity cannot be collected with the upper respiratory tract. Collect the sample by letting the spherical travel touch the part near the posterior pharyngeal wall safely to swab a part near the lower respiratory tract. Also, do not use nasopharyngeal swabs when collecting samples as it may cause insufficient sample collection.
4. All samples must be tested as soon as they are collected.
5. In case of direct sampling (with nasopharyngeal/oropharyngeal swab), the extraction solution containing the sample can be stored at room temperature for up to 1 hour or at 2-8°C (36-46°F) for a maximum of 1 hour. maximum of 12 hours before testing In case of using VTM/UTM samples, avoid multiple freeze/thaw cycles.
Guide for STANDARD Q Ag Home Test
2 Reviews Hide Reviews Show Reviews
-
Fast delivery
The parcel was very finely packed and the shipping was on time. highly suggestable!
-
Outstanding
The items are extremely viable just as different administrations of the organization as well. Great work and progress Gentaur. Keep it up.